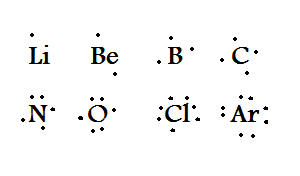Ace Info About How To Draw Electron Dot Diagrams

Oxygen is in group vi of the periodic.
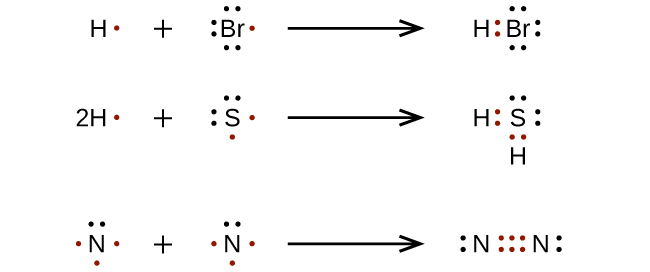
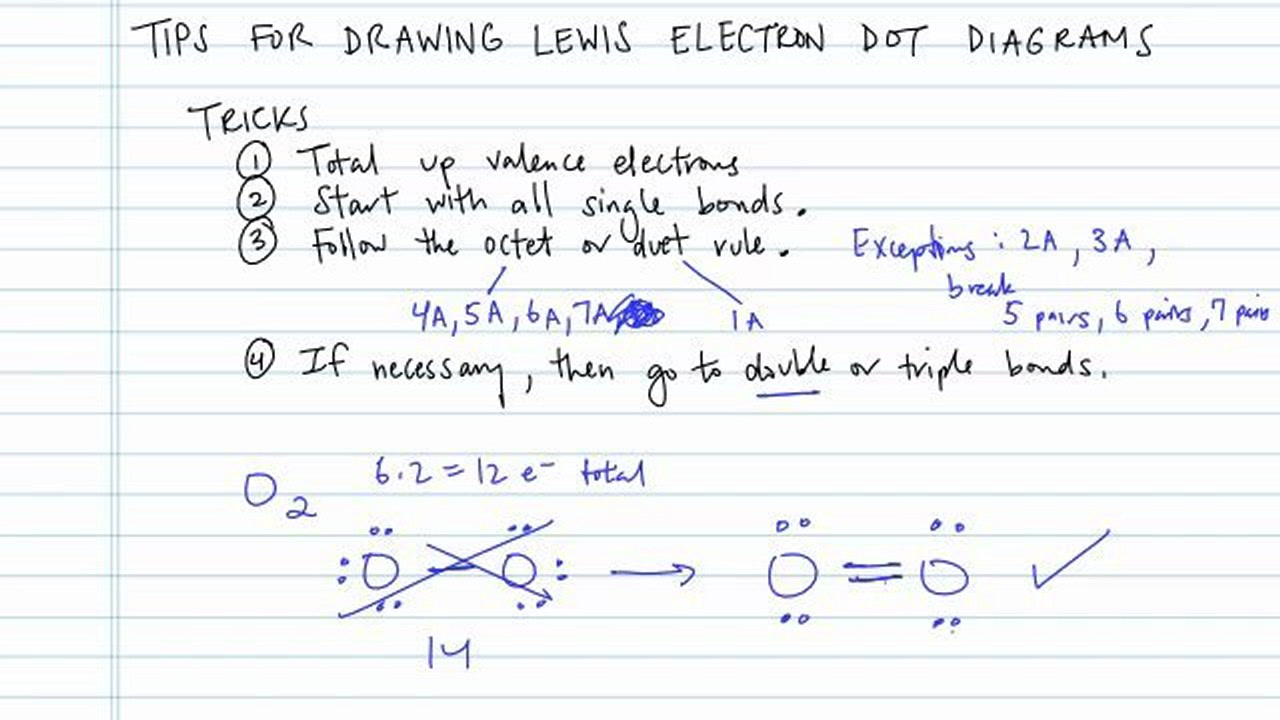
How to draw electron dot diagrams. Add single covalent bonds to. Place one electron pair between. Andersen shows you how to draw lewis dot diagrams for atoms and simple molecules.intro music atributiontitle:
Draw the rough position of the atoms in the molecule. Write the element name in the center surrounding by the number of valence electrons around. Draw the lewis electron dot diagram.
Find the total number of valence electrons; 3) you should have 4 total electrons, or dots, drawn in for carbon. Find the number of electrons that would satisfy the outermost shell;
Count the number of valence electrons in the molecule. Electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. Use the accompanying fact sheet and worksheet to get your students drawing electron configuration diagrams.
A beryllium atom, with two valence electrons, would. For cations subtract a number of electrons equal to the positive charge. To draw lewis dot diagrams, follow these steps:
For anions add a number of electrons equal to the negative charge. Draw the lewis electron dot diagram for each element. An electron dot diagram is a lewis dot diagram.






/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)